How Long Will a 1200mah Lipo Battery Last in Airsoft
How Long Will a 1200mah Lipo Battery Last in Airsoft
 A lithium polymer battery used to power a smartphone | |
| Specific free energy | 100–265 W·h/kg (0.36–0.95 MJ/kg) [1] |
|---|---|
| Energy density | 250–670 W·h/L (0.xc–2.63 MJ/L) [ii] |
A lithium polymer battery, or more correctly lithium-ion polymer battery (abbreviated as LiPo, LIP, Li-poly, lithium-poly and others), is a rechargeable battery of lithium-ion technology using a polymer electrolyte instead of a liquid electrolyte. High conductivity semisolid (gel) polymers form this electrolyte. These batteries provide higher specific energy than other lithium battery types and are used in applications where weight is a critical feature, such as mobile devices, radio-controlled shipping and some electric vehicles.[3]
History [edit]
LiPo cells follow the history of lithium-ion and lithium-metallic cells which underwent all-encompassing research during the 1980s, reaching a significant milestone with Sony's beginning commercial cylindrical Li-ion cell in 1991. After that, other packaging forms evolved, including the flat pouch format.[iv]
Design origin and terminology [edit]
Lithium polymer cells accept evolved from lithium-ion and lithium-metal batteries. The primary difference is that instead of using a liquid lithium-salt electrolyte (such as LiPF6) held in an organic solvent (such as EC/DMC/DEC), the battery uses a solid polymer electrolyte (SPE) such as poly(ethylene oxide) (PEO), poly(acrylonitrile) (PAN), poly(methyl methacrylate) (PMMA) or poly(vinylidene fluoride) (PVdF).
The solid electrolyte can typically be classified equally one of three types: dry SPE, gelatinous SPE and porous SPE. The dry out SPE was the starting time used in image batteries, around 1978 by Michel Armand,[five] [six] and 1985 past ANVAR and Elf Aquitaine of France, and Hydro Quebec of Canada.[7] From 1990 several organisations like Mead and Valence in the U.s. and GS Yuasa in Japan developed batteries using gelled SPEs.[7] In 1996, Bellcore in the United states announced a rechargeable lithium polymer cell using porous SPE.[7]
A typical prison cell has 4 principal components: positive electrode, negative electrode, separator and electrolyte. The separator itself may exist a polymer, such as a microporous film of polyethylene (PE) or polypropylene (PP); thus, fifty-fifty when the cell has a liquid electrolyte, it will withal comprise a "polymer" component. In addition to this, the positive electrode tin be further divided into iii parts: the lithium-transition-metal-oxide (such as LiCoO2 or LiMn2Ofour), a conductive additive, and a polymer folder of poly(vinylidene fluoride) (PVdF).[8] [9] The negative electrode material may have the same 3 parts, just with carbon replacing the lithium-metal-oxide.[8] [9]
Working principle [edit]
Merely as with other lithium-ion cells, LiPos work on the principle of intercalation and de-intercalation of lithium ions from a positive electrode fabric and a negative electrode material, with the liquid electrolyte providing a conductive medium. To prevent the electrodes from touching each other straight, a microporous separator is in betwixt which allows only the ions and non the electrode particles to drift from one side to the other.
Voltage and state of charge [edit]
The voltage of a single LiPo jail cell depends on its chemistry and varies from about 4.2 Five (fully charged) to about two.7–3.0 5 (fully discharged), where the nominal voltage is 3.6 or iii.vii volts (about the middle value of highest and lowest value) for cells based on lithium-metal-oxides (such as LiCoO2). This compares to 3.vi–3.8 V (charged) to one.8–2.0 V (discharged) for those based on lithium-iron-phosphate (LiFePO4).
The verbal voltage ratings should be specified in product data sheets, with the understanding that the cells should exist protected by an electronic excursion that won't allow them to overcharge nor over-discharge nether utilize.
LiPo battery packs, with cells connected in series and parallel, take separate pivot-outs for every jail cell. A specialized charger may monitor the charge on a per-cell footing and so that all cells are brought to the aforementioned state of charge (SOC).
Applying force per unit area on LiPo cells [edit]
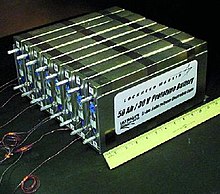
An experimental lithium-ion polymer battery made by Lockheed-Martin for NASA
Unlike lithium-ion cylindrical and prismatic cells, which have a rigid metal case, LiPo cells accept a flexible, foil-type (polymer laminate) instance, and then they are relatively unconstrained. Moderate pressure on the stack of layers that etch the cell results in increased capacity retention, because the contact between the components is maximised and delamination and deformation is prevented, which is associated with increase of cell impedance and degradation. [ten] [11]
Applications [edit]

Hexagonal lithium polymer bombardment for underwater vehicles made by Custom Cells Itzehoe GmbH
LiPo cells provide manufacturers with compelling advantages. They tin hands produce batteries of almost any desired shape. For example, the space and weight requirements of mobile devices and notebook computers tin can exist met. They too have a low self-belch rate, which is most 5% per calendar month.[12]
Drones, Radio controlled equipment and aircraft [edit]

LiPo batteries are now near ubiquitous when used to power commercial and hobby drones (unmanned_aerial_vehicles), radio-controlled shipping, radio-controlled cars and large scale model trains, where the advantages of lower weight and increased capacity and power delivery justify the price. Test reports warn of the risk of fire when the batteries are not used in accordance with the instructions.[xiii]
The voltage for long-time storage of LiPo battery used in the R/C model should be iii.6~3.9V range per cell, otherwise it may crusade impairment to the battery.[14]
LiPo packs also see widespread utilise in airsoft, where their higher discharge currents and better free energy density compared to more traditional NiMH batteries has very noticeable operation gain (higher charge per unit of burn). The high discharge currents practice damage the switch contacts due to arcing (causing the contacts to oxidize and oftentimes deposit carbon), so it is advised to either use a solid-state MOSFET switch or clean the trigger contacts regularly.
Personal electronics [edit]
LiPo batteries are pervasive in mobile devices, power banks, very thin laptop computers, portable media players, wireless controllers for video game consoles, wireless PC peripherals, electronic cigarettes, and other applications where small-scale form factors are sought and the high free energy density outweighs cost considerations.
Electric vehicles [edit]
Hyundai Motor Company uses this type of battery in some of its battery electric and hybrid vehicles,[15] as well as Kia Motors in their battery electric Kia Soul.[16] The Bolloré Bluecar, which is used in car sharing schemes in several cities, also uses this blazon of battery.
Uninterruptible power supply systems [edit]
Lithium-ion batteries are becoming increasingly more commonplace in Uninterruptible power supply (UPS) systems. They offer numerous benefits over the traditional VRLA battery and with stability and safety improvements confidence in the engineering science is growing. Their power to size and weight ratio is seen as a major benefit in many industries requiring disquisitional power support including data centers where space is frequently at a premium.[17] The longer cycle life, usable energy (Depth of discharge), and thermal delinquent are also seen equally a benefit for using Li-po batteries over VRLA batteries.
Spring starter [edit]
The battery used to first a vehicle engine is typically 12V or 24V, and so a portable bound starter or battery booster uses three or vi LiPo batteries in series (3S1P/6S1P) to start the vehicle in an emergency, instead of the other leap-start methods. The price of a pb-acid jump starter is less but they are bigger and heavier than comparable lithium batteries, and so such products have mostly switched to LiPo batteries or sometimes lithium fe phosphate batteries.
Safety [edit]

Apple iPhone 3GS's Lithium-ion battery, which has expanded due to a short excursion failure.
All Li-ion cells expand at high levels of state of charge (SOC) or over-charge, due to slight vaporisation of the electrolyte. This may result in delamination, and thus bad contact of the internal layers of the cell, which in turn brings macerated reliability and overall cycle life of the cell.[10] This is very noticeable for LiPos, which can visibly inflate due to lack of a hard case to contain their expansion. The safety characteristics of Lithium Polymer batteries are different from those of lithium fe phosphate batteries .
Lithium cells with solid polymer electrolyte [edit]
Cells with solid polymer electrolytes have non reached full commercialization[18] and are nonetheless a topic of inquiry.[nineteen] Prototype cells of this blazon could be considered to be between a traditional lithium-ion battery (with liquid electrolyte) and a completely plastic, solid-state lithium-ion battery.[20]
The simplest approach is to use a polymer matrix, such as polyvinylidene fluoride (PVdF) or poly(acrylonitrile) (PAN), gelatinous with conventional salts and solvents, such as LiPF6 in EC/DMC/DEC.
Nishi mentions that Sony started research on lithium-ion cells with gelled polymer electrolytes (GPE) in 1988, before the commercialisation of the liquid-electrolyte lithium-ion cell in 1991.[21] At that time polymer batteries were promising and it seemed polymer electrolytes would get indispensable.[22] Eventually, this type of cell went into the market place in 1998.[21] However, Scrosati argues that, in the strictest sense, gelled membranes cannot be classified as "true" polymer electrolytes, but rather every bit hybrid systems where the liquid phases are contained within the polymer matrix.[xx] Although these polymer electrolytes may exist dry to the touch, they can still contain 30% to 50% liquid solvent.[23] In this regard, how to really define what a "polymer battery" is remains an open question.
Other terms used in the literature for this system include hybrid polymer electrolyte (HPE), where "hybrid" denotes the combination of the polymer matrix, the liquid solvent and the salt.[24] It was a system like this that Bellcore used to develop an early lithium-polymer cell in 1996,[25] which was called "plastic" lithium-ion jail cell (PLiON), and afterward commercialised in 1999.[24]
A solid polymer electrolyte (SPE) is a solvent-free salt solution in a polymer medium. It may exist, for case, a compound of lithium bis(fluorosulfonyl)imide (LiFSI) and high molecular weight poly(ethylene oxide) (PEO),[26] or a high molecular weight poly(trimethylene carbonate) (PTMC).[27]
The performance of these proposed electrolytes is unremarkably measured in a half-cell configuration against an electrode of metal lithium, making the system a "lithium-metallic" cell, but it has also been tested with a common lithium-ion cathode material such as lithium-fe-phosphate (LiFePOiv).
Other attempts to pattern a polymer electrolyte cell include the use of inorganic ionic liquids such as 1-butyl-three-methylimidazolium tetrafluoroborate ([BMIM]BFiv) equally a plasticizer in a microporous polymer matrix like poly(vinylidene fluoride-co-hexafluoropropylene)/poly(methyl methacrylate) (PVDF-HFP/PMMA).[28]
See also [edit]
- List of battery types
- Lithium–air battery
- Lithium fe phosphate battery
- Research in lithium-ion batteries
References [edit]
- ^ "Lithium-Ion Battery". Clean Energy Found . Retrieved 6 January 2022.
- ^ "Lithium-Ion Battery". Make clean Free energy Constitute . Retrieved 6 January 2022.
- ^ Bruno Scrosati, Chiliad. One thousand. Abraham, Walter A. van Schalkwijk, Jusef Hassoun (ed), Lithium Batteries: Advanced Technologies and Applications, John Wiley & Sons, 2013 ISBN 1118615395,folio 44
- ^ "Lithium Battery Configurations and Types of Lithium Cells". Power Sonic. 25 March 2021. Retrieved xiv October 2021.
- ^ Yard. B. Armand; J. M. Chabagno; 1000. Duclot (20–22 September 1978). "Extended Abstracts". Second International Meeting on Solid Electrolytes. St. Andrews, Scotland.
- ^ M. B. Armand, J. Yard. Chabagno & M. Duclot (1979). "Poly-ethers as solid electrolytes". In P. Vashitshta; J.Due north. Mundy & Grand.K. Shenoy (eds.). Fast ion Transport in Solids. Electrodes and Electrolytes. North Holland Publishers, Amsterdam.
- ^ a b c Murata, Kazuo; Izuchi, Shuichi; Yoshihisa, Youetsu (3 January 2000). "An overview of the research and development of solid polymer electrolyte batteries". Electrochimica Acta. 45 (viii–9): 1501–1508. doi:ten.1016/S0013-4686(99)00365-v.
- ^ a b Yazami, Rachid (2009). "Chapter 5: Thermodynamics of Electrode Materials for Lithium-Ion Batteries". In Ozawa, Kazunori (ed.). Lithium ion rechargeable batteries. Wiley-Vch Verlag GmbH & Co. KGaA. ISBN978-3-527-31983-1.
- ^ a b Nagai, Aisaku (2009). "Chapter half-dozen: Applications of Polyvinylidene Fluoride-Related Materials for Lithium-Ion Batteries". In Yoshio, Masaki; Brodd, Ralph J.; Kozawa, Akiya (eds.). Lithium-ion batteries. Springer. Bibcode:2009liba.book.....Y. doi:x.1007/978-0-387-34445-iv. ISBN978-0-387-34444-7.
- ^ a b Vetter, J.; Novák, P.; Wagner, Yard.R.; Veit, C. (ix September 2005). "Ageing mechanisms in lithium-ion batteries". Periodical of Power Sources. 147 (1–2): 269–281. Bibcode:2005JPS...147..269V. doi:10.1016/j.jpowsour.2005.01.006.
- ^ Cannarella, John; Arnold, Craig B. (1 January 2014). "Stress evolution and capacity fade in constrained lithium-ion pouch cells". Journal of Power Sources. 245: 745–751. Bibcode:2014JPS...245..745C. doi:10.1016/j.jpowsour.2013.06.165.
- ^ "Lithium Polymer Bombardment Technology" (PDF) . Retrieved fourteen March 2016.
- ^ Dunn, Terry (5 March 2015). "Battery Guide: The Nuts of Lithium-Polymer Batteries". Tested. Whalerock Industries. Retrieved 15 March 2017.
I've not still heard of a LiPo that burst into flames during storage. All of the fire incidents that I'k aware of occurred during accuse or discharge of the battery. Of those cases, the majority of issues happened during charge. Of those cases, the fault usually rested with either the charger or the person who was operating the charger…simply not always.
- ^ "A LIPO Battery GUIDE TO Sympathize LIPO BATTERY". Retrieved 3 September 2021.
- ^ Brown, Warren (3 November 2011). "2011 Hyundai Sonata Hybrid: Hi, tech. Cheerio, performance". Washington Post . Retrieved 25 November 2011.
- ^ "Sustainability | Kia Global Brand Site".
- ^ "Lithium-ion vs Lithium Fe: Which is the most suitable for a UPS system?".
- ^ Blain, Loz (27 November 2019). "Solid state battery quantum could double the density of lithium-ion cells". New Atlas. Gizmag. Retrieved six December 2019.
- ^ Wang, Xiaoen; Chen, Fangfang; Girard, Gaetan 1000.A.; Zhu, Haijin; MacFarlane, Douglas R.; Mecerreyes, David; Armand, Michel; Howlett, Patrick C.; Forsyth, Maria (November 2019). "Poly(Ionic Liquid)southward-in-Salt Electrolytes with Co-coordination-Assisted Lithium-Ion Transport for Condom Batteries". Joule. three (11): 2687–2702. doi:10.1016/j.joule.2019.07.008.
- ^ a b Scrosati, Bruno (2002). "Chapter eight: Lithium polymer electrolytes". In van Schalkwijk, Walter A.; Scrosati, Bruno (eds.). Advances in Lithium-ion batteries. Kluwer Bookish Publishers. ISBN0-306-47356-9.
- ^ a b Yoshio, Masaki; Brodd, Ralph J.; Kozawa, Akiya, eds. (2009). Lithium-ion batteries. Springer. Bibcode:2009liba.book.....Y. doi:10.1007/978-0-387-34445-iv. ISBN978-0-387-34444-7.
- ^ Nishi, Yoshio (2002). "Chapter 7: Lithium-Ion Secondary batteries with gelled polymer electrolytes". In van Schalkwijk, Walter A.; Scrosati, Bruno (eds.). Advances in Lithium-ion batteries. Kluwer Academic Publishers. ISBN0-306-47356-9.
- ^ Brodd, Ralf J. (2002). "Chapter 9: Lithium-Ion prison cell production processes". In van Schalkwijk, Walter A.; Scrosati, Bruno (eds.). Advances in Lithium-ion batteries. Kluwer Bookish Publishers. ISBN0-306-47356-ix.
- ^ a b Tarascon, Jean-Marie; Armand, Michele (2001). "Bug and challenges facing rechargeable lithium batteries". Nature. 414 (6861): 359–367. Bibcode:2001Natur.414..359T. doi:10.1038/35104644. PMID 11713543. S2CID 2468398.
- ^ Tarascon, J.-M.; Gozdz, A. S.; Schmutz, C.; Shokoohi, F.; Warren, P. C. (July 1996). "Performance of Bellcore's plastic rechargeable Li-ion batteries". Solid State Ionics. Elsevier. 86–88 (Role 1): 49–54. doi:10.1016/0167-2738(96)00330-X.
- ^ Zhang, Heng; Liu, Chengyong; Zheng, Liping (1 July 2014). "Lithium bis(fluorosulfonyl)imide/poly(ethylene oxide) polymer electrolyte". Electrochimica Acta. 133: 529–538. doi:ten.1016/j.electacta.2014.04.099.
- ^ Sun, Bing; Mindemark, Jonas; Edström, Kristina; Brandell, Daniel (1 September 2014). "Polycarbonate-based solid polymer electrolytes for Li-ion batteries". Solid State Ionics. 262: 738–742. doi:ten.1016/j.ssi.2013.08.014.
- ^ Zhai, Wei; Zhu, Hua-jun; Wang, Long (1 July 2014). "Study of PVDF-HFP/PMMA composite micro-porous gel polymer electrolyte incorporating ionic liquid [BMIM]BFfour for Lithium ion batteries". Electrochimica Acta. 133: 623–630. doi:10.1016/j.electacta.2014.04.076.
External links [edit]
- Electropaedia on Lithium Battery Manufacturing
- Electropaedia on Lithium Bombardment Failures
How Long Will a 1200mah Lipo Battery Last in Airsoft
Posted by: caudillworsir.blogspot.com
0 Response to "How Long Will a 1200mah Lipo Battery Last in Airsoft"
Post a Comment